14 Days CGM Sugar Test Machine – Needleless Glucose Monitor & Blood Sugar Testing Device
What’s Included in the 14-Day CGM Sugar Test Kit
-
Continuous Glucose Monitor (CGM) Sensor (Sensor works for 14 days)
-
Sensor Application, Readings and Removal at Home
-
Turn Around Time: 24 Hrs
About the Continuous Glucose Monitoring (CGM) Device
The Freestyle Libre Pro Continuous Glucose Monitoring (CGM) Sensor (FDA Approved) allows your healthcare provider to place a small sensor at the back of your upper arm that records up to 14 days of continuous glucose data.
The provider then downloads the data revealing personalised glycaemic profile, trends, and patterns that can be used for diabetes management or to monitor your diabetes, including values from interstitial fluid. This device is known for its reliability in providing accurate results. Continuous glucose monitors (CGMs) play a crucial role in diabetes management by enhancing the understanding of glucose levels and supporting clinical research. The Freestyle Libre sugar test machine is ideal for individuals that do not prefer the finger prick method of measuring levels. If the current sensor fails or is removed, having a new sensor is essential to continue effective glucose monitoring.
You can get our continuous blood sugar monitoring tests across major cities in India. Our testing coverage includes Bangalore, Hyderabad, Chennai, Mumbai, Pune, Ahmedabad, Delhi, Gurgaon, Noida, Ghaziabad, Faridabad, Jaipur, Lucknow, Kolkata, and Chandigarh.
Real-Time Sugar Monitoring with Needle-Free CGM Sensors
The New-Age metrics beyond fasting blood sugar and HbA1c, which is provided by CGM
-
Glycemic Variability
-
Time-in-Range
-
Risk of Hypo and Hyper Glycemic Episodes
-
Glucose Management Indicator (GMI)
CGM devices provide blood glucose readings in real-time by measuring glucose levels in the interstitial fluid. This allows for continuous monitoring and better diabetes management.
The device measures glucose levels continuously, offering a comprehensive view of glucose trends and patterns.
How Accurate Are Continuous Glucose Monitoring Devices?
-
CGM systems are generally considered to be accurate, but they may not be as accurate as traditional blood glucose monitors. Several factors can affect the accuracy of CGM devices, including the placement of the sensor, the type of sensor used, and the condition of the skin where the sensor is inserted.
-
Freestyle Libre CGM systems does not require calibration and it ensures that the sensor is accurately measuring the glucose levels in the interstitial fluid.
Who Should Use a CGM Device: Diabetes, Weight Loss & Fitness
Manage & Reverse Diabetes Using a Continuous Sugar Monitor
You can monitor your blood sugar levels in real time with the continuous glucose monitoring machine, which is essential for managing diabetes. You can bring your A1c levels within range, as well as track advanced metrics beyond fasting sugar. You can also track your history of sugar levels and view CGM data through the Freestyle Libre. It is crucial to follow a personalized treatment plan recommended by your healthcare provider to effectively manage your blood sugar levels.
Use CGM to Tailor Nutrition & Track Glucose for Weight Loss
A CGM machine can help you understand the best approach for you to focus on weight management. Whether doctor advised or self motivated, weight loss can be challenging when you have diabetes or issues with low blood sugar. You can customize a highly detailed plan and measure your levels with the sugar machine. This can help prevent spikes and crashes for optimal weight management.
Track Sugar for Better Metabolic Health with CGM
The Freestyle Libre sugar check machine can give you instant insights on your sugar levels so that you can avoid certain foods and optimize your metabolic health. You can also ensure that your levels are always within range.
Monitor Glucose During Exercise with a Patch-Based CGM
By ensuring that your sugar levels are always within normal range through the Freestyle diabetes machine for tracking, you can optimize energy generation and recovery. You can get scientific insights on your body's response to athletic input and muscle building, as well as access to glucose information .

CGM vs Glucometer: Which Sugar Monitor is Right for You?
below the skin. CGM systems measure glucose in the interstitial fluid – the fluid between your cells just under the skin. Since CGM is measuring glucose in a different part of the body, it will not be the same concentration as in the blood.
A blood glucose meter (BGM) takes a small sample of blood from your fingertip and places it on a test strip that’s inserted inside a meter. The meter provides a glucose value based on the amount of glucose in the blood sample at that moment. Traditional blood glucose meters require a drop of blood for each test.
CGM devices eliminate the need for frequent blood samples, offering a more convenient and less invasive method for monitoring glucose levels.
How a CGM Sensor Works to Track Blood Sugar 24/7
The CGM sensor is a very thin wire or filament, inserted with the aid of a needle under the skin. This glucose sensor is applied on the back of your arm. In order to hold the sensor in place, there’s a sticky backing like a band-aid, that attaches the sensor to the skin. Using similar enzymes as a test strip for a glucose meter, the sensor’s job is to monitor glucose in the interstitial fluid, which is the fluid between the cells. Sensors can stay in place for several days (about 14 days).
Certain implantable sensors can last significantly longer, up to 180 days, offering advancements in CGM technology and addressing potential user concerns about sensor accuracy and maintenance.
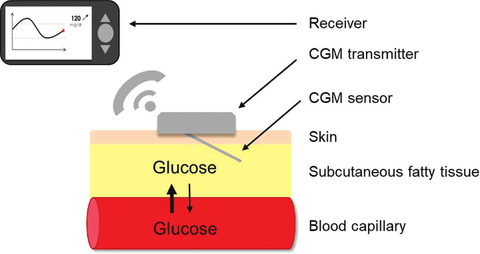
Most CGMs operate by detecting glucose levels through interstitial fluids in the skin, providing a convenient alternative to traditional blood glucose meters. They also include components like sensors and transmitters, emphasizing the wireless functionality that allows users to receive glucose data on their smartphones.
Top Benefits of Using a Continuous Glucose Monitor
-
Provides 14 days of continuous glucose readings. The sensor takes a reading every 15 minutes, so in a day it takes 96 readings (even while you are sleeping). Total 1344 readings provide a high-resolution glucose profile & trends. The special features of CGM devices, such as the ability to work with apps for additional tracking capabilities, enhance their functionality and improve safety and ease of use.
-
This is the only device which records your glucose levels while you are asleep thus measuring the key data during hypoglycaemic episodes. The storage capacity of CGM devices allows for convenient data recording directly on the device, enabling users to retain health readings efficiently and improving the functionality and usability of the monitors.
-
No multiple pricking … Just install once and keep up with your daily activity while the sensor does its job

THIS IS A MEDICAL GRADE DEVICE TO BE INSTALLED AND SUPERVISED BY A REGISTERED MEDICAL PRACTITIONER ONLY.
Complete details of the sensor are available at
https://www.myfreestyle.com/freestyle-libre-pro-cgm-system
How to Prepare Before Using a CGM Sugar Test Machine
-
There are no-prerequisite for the test
How to Book and Install Your CGM Device at Home
-
Once you book the sensor, you can come to our clinic to install the sensor or you can buy the entire kit. The installation will be done by the qualified practitioner upon doctor review or you can self install at-home
-
You can come in-between to check the readings and on the 15th day, come to the clinic to remove the sensor. You can read the readings on your own through readers.
-
Once the sensor is removed, the report can be accessed through readers





